Body Composition
(30) THE EFFECT OF HYDRATION STATUS AND MUSCLE GLYCOGEN ON MUSCLE SIZE AND ECHO INTENSITY

Matthew LeMay
Research Assistant
Creighton University
Fort Myers, Florida, United States
April E. Krywe (she/her/hers)
Undergraduate
Creighton University
Omaha, Nebraska, United States- JS
Jacob Siedlik
Associate Professor
Creighton University
Omaha, Nebraska, United States 
Mitchel A. Magrini, PhD
Assistant Professor
Creighton University
Omaha, Nebraska, United States- MK
Melani Kelly
Assistant Professor
Utah Valley University
Orem, Utah, United States
Poster Presenter(s)
Author(s)
Diagnostic Ultrasonography is becoming more popular to examine skeletal muscle architecture. P<span style="color: black; mso-themecolor: text1;">revious research has suggested that hydration status and muscle glycogen content may influence the muscle’s architecture leading to inaccurate outcomes. However, limited research has examined the impact that hydration and muscle glycogen content have on skeletal muscle size and brightness.
Purpose: To determine the effects of hydration status and muscle glycogen content on ultrasonography derived muscle size and echo intensity.
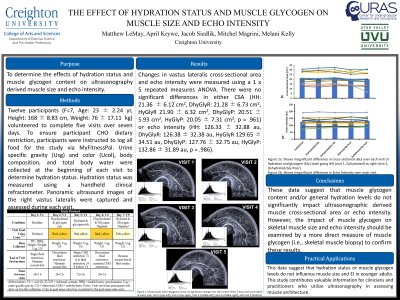
Methods: Twelve participants (F=7, Age: 23 ± 2.24 yr, Height: 168 ± 8.83 cm, Weight: 76 ± 17.11 kg) volunteered to complete five visits over seven days. Visit 1 served as participant’s habitual hydration (HH) level condition. Following the first visit, participants were instructed to return for visit two following a 24-hour fluid restriction to serve as the dehydrated and glycogen rich condition (DhyGlyR). Following the second visit, participants were instructed to discontinue fluid restriction and maintain a normal diet to serve as the hydrated and glycogen rich condition (HyGlyR). During Visit 3, participants completed a glycogen depletion protocol consisting of repeated 30s:30s work to rest sprints until volitional exhaustion. When participants completed the fatigue protocol, they were instructed to start a 72-hour dietary restriction of less than 75g of CHO/day. After 48 hours, participants were instructed to start a fluid restriction before visit 4 to serve as the dehydrated and glycogen poor condition (DhyGlyP). Following visit 4, participants were instructed to discontinue the fluid restriction but continue the CHO restriction for 24 hours after visit 4 to allow visit 5, final visit, to serve as a hydrated and glycogen poor condition (HyGlyP). To ensure participant CHO dietary restriction, participants were instructed to log all food for the study via MyFitnessPal. Urine specific gravity (Usg) and color (Ucol), body composition, and total body water were collected at the beginning of each visit to determine hydration status. Hydration status was measured using a handheld clinical refractometer. Panoramic ultrasound images of the right vastus lateralis were captured and assessed during each visit. Changes in vastus lateralis cross-sectional area and echo intensity were measured using a 1 x 5 repeated measures ANOVA.
Results: There were no significant differences in either CSA (HH: 21.36 ± 6.12 cm2, DhyGlyR: 21.28 ± 6.73 cm2, HyGlyR 21.90 ± 6.32 cm2, DhyGlyP: 20.51 ± 5.93 cm2, HyGlyP: 20.05 ± 7.31 cm2, p = .961) or echo intensity (HH: 126.33 ± 32.88 au, DhyGlyR: 126.38 ± 32.38 au, HyGlyR 129.65 ± 34.51 au, DhyGlyP: 127.76 ± 32.75 au, HyGlyP: 132.86 ± 31.89 au, p = .986).
Conclusions: These data suggest that muscle glycogen content and/or general hydration levels do not significantly impact ultrasonographic derived muscle cross-sectional area or echo intensity. However, the impact of muscle glycogen on skeletal muscle size and echo intensity should be examined by a more direct measure of muscle glycogen (i.e., skeletal muscle biopsy) to confirm these results. Practical Applications: These data suggest that hydration status or muscle glycogen levels do not influence muscle size and EI in younger adults. This study contributes valuable information for clinicians and practitioners who utilize ultrasonography in assessing muscle architecture.
Acknowledgements: MIRA
